-
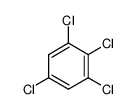
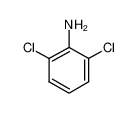
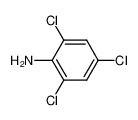
2,4,6-Trichloroaniline
CAS No.634-93-5
Formula:C6H4Cl3N
Formula
C6H4Cl3N
Molecular Weight
196.462
Exact Mass
194.941
LogP
3.8102
PSA
26.02
Synonyms
2,4,6-Trichlorophenylamine
1-amino-2,4,6-trichlorobenzene
Aniline,2,4,6-trichloro
sym-Trichloroaniline
2,4,6-Trichlor-anilin
2,3-DIHYDROBENZO[1,4]DIOXINE-5-CARBONYL CHLORIDE 0.98
Benzenamine, 2,4,6-trichloro-
2,4,6-trichloro-aniline
2,4,6-Trichlorobenzenamine
s-Trichloroaniline
2,4,6-trichloraniline
Benzenamine,2,4,6-trichloro
expand collapse
Appearance & Physical State
White Crystal
Density
1.54 g/cm3
Boiling Point
262ºC
Melting Point
73-75ºC
Flash Point
110.1ºC
Stability
Stable, but may be light or air sensitive. Incompatible with acids, acid chlorides, acid anhydrides, chloroformates, strong oxidizing agents.
Storage Condition
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Poison room locked.
SDS 1.0
expand
collapse
MSDS
expand
collapse
| Name: | 2 4 6-Trichloroaniline 98.5% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 634-93-5 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 634-93-5 | 2,4,6-Trichloroaniline | 98.5 | 211-219-8 |
Risk Phrases: 23/24/25 33 50/53
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Toxic by inhalation, in contact with skin and if swallowed. Danger of cumulative effects. Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. May cause cardiac disturbances. May cause methemoglobinemia, cyanosis (bluish discoloration of skin due to deficient oxygenation of the blood), convulsions, and death. The toxicological properties of this substance have not been fully investigated. May cause central nervous system depression.
Inhalation:
May cause respiratory tract irritation. May cause methemoglobinemia, cyanosis (bluish discoloration of skin due to deficient oxygenation of the blood), convulsions, tachycardia, dyspnea (labored breathing), and death. The toxicological properties of this substance have not been fully investigated. May cause cardiac abnormalities. Inhalation at high concentrations may cause CNS depression and asphixiation.
Chronic:
May cause methemoglobinemia, which is characterized by chocolate-brown colored blood, headache, weakness, dizziness, breath shortness, cyanosis (bluish skin due to deficient oxygenation of blood), rapid heart rate, unconsciousness and possible death.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
For methemoglobinemia, administer oxygen alone or with Methylene Blue depending on the methemoglobin concentration in the blood.
Antidote: Methylene blue, alone or in combination with oxygen is indicated as a treatment in nitrite induced methemoglobinemia.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Containers may explode when heated. Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam. Use water spray to cool fire-exposed containers. Do NOT get water inside containers. For small fires, use dry chemical, carbon dioxide, or water spray. For large fires, use dry chemical, carbon dioxide, alcohol-resistant foam, or water spray.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Poison room locked.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 634-93-5: Personal Protective Equipment Eyes: Wear safety glasses and chemical goggles if splashing is possible. Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves and clothing to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: beige
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 262 deg C @ 760.00mmHg
Freezing/Melting Point: 76.00 - 79.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: insoluble
Specific Gravity/Density:
Molecular Formula: C6H4Cl3N
Molecular Weight: 196.46
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents, acids, acid anhydrides, chloroformates, acid chlorides.
Hazardous Decomposition Products:
Hydrogen chloride, nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 634-93-5: BZ0250000 LD50/LC50:
CAS# 634-93-5: Oral, mouse: LD50 = 1180 mg/kg; Oral, rat: LD50 = 2400 mg/kg.
Carcinogenicity:
2,4,6-Trichloroaniline - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Other No information available.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2811
Packing Group: II
IMO
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: II
RID/ADR
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: T N
Risk Phrases:
R 23/24/25 Toxic by inhalation, in contact with skin
and if swallowed.
R 33 Danger of cumulative effects.
R 50/53 Very toxic to aquatic organisms, may cause
long-term adverse effects in the aquatic environment.
Safety Phrases:
S 28A After contact with skin, wash immediately with
plenty of water.
S 36/37 Wear suitable protective clothing and
gloves.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
S 60 This material and its container must be
disposed of as hazardous waste.
S 61 Avoid release to the environment. Refer to
special instructions/safety data sheets.
WGK (Water Danger/Protection)
CAS# 634-93-5: 3
Canada
CAS# 634-93-5 is listed on Canada's NDSL List.
CAS# 634-93-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 634-93-5 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Van Den Dool and Kratz RI, non-polar column, custom temperature program
expand
collapse
Column Shape
Capillary
Active Phase(℃)
5 % Phenyl methyl siloxane
Retention index
1398.
Temperature Control
Method
custom temperature program
Comments
25. m/0.31 mm/0.52 μm, He; Program: 50C(2min) => (20C/min) => 120C => (7C/min) => 310C(10min)
Reference
Yasuhara, A.Shiraishi, H.Nishikawa, M.Yamamoto, T.Uehiro, T.Nakasugi, O.Okumura, T.Kenmotsu, K.Fukui, H.Nagase, M.Ono, Y.Kawagoshi, Y.Baba, K.Noma, Y.Determination of organic components in leachates from hazardous waste disposal sites in Japan by gas chromatography-mass spectrometryJ. Chromatogr. A1997, 774, 1-2, 321-332.
Normal alkane RI, non-polar column, temperature ramp
expand
collapse
Column Shape
Capillary
Active Phase(℃)
TR-1
Retention index
1367.
Temperature Control
Method
temperature ramp
Comments
30. m/0.32 mm/0.25 μm, Helium, 5. K/min; T<sub>start</sub>: 50. C; T<sub>end</sub>: 300. C
Reference
Gruzdev, I.V.Alferova, M.V.Kondratenok, B.M.Zenkevich, I.G.Gas-chromatographic identification of chloro- and bromosubstituted anilines using retention indicesRus. J. Anal. Chem.2011, 66, 5, 519-524.
Normal alkane RI, non-polar column, temperature ramp
expand
collapse
Column Shape
Capillary
Active Phase(℃)
TR-1
Retention index
1371.
Temperature Control
Method
temperature ramp
Comments
30. m/0.32 mm/0.25 μm, Helium, 5. K/min; T<sub>start</sub>: 50. C; T<sub>end</sub>: 300. C
Reference
Gruzdev, I.V.Alferova, M.V.Kondratenok, B.M.Zenkevich, I.G.Gas-chromatographic identification of chloro- and bromosubstituted anilines using retention indicesRus. J. Anal. Chem.2011, 66, 5, 519-524.
Normal alkane RI, non-polar column, temperature ramp
expand
collapse
Column Shape
Capillary
Active Phase(℃)
TR-1
Retention index
1379.
Temperature Control
Method
temperature ramp
Comments
30. m/0.32 mm/0.25 μm, Helium, 5. K/min; T<sub>start</sub>: 50. C; T<sub>end</sub>: 300. C
Reference
Gruzdev, I.V.Alferova, M.V.Kondratenok, B.M.Zenkevich, I.G.Gas-chromatographic identification of chloro- and bromosubstituted anilines using retention indicesRus. J. Anal. Chem.2011, 66, 5, 519-524.
Normal alkane RI, non-polar column, temperature ramp
expand
collapse
Column Shape
Capillary
Active Phase(℃)
TR-1
Retention index
1367.
Temperature Control
Method
temperature ramp
Comments
30. m/0.32 mm/0.25 μm, Helium, 5. K/min; T<sub>start</sub>: 50. C; T<sub>end</sub>: 300. C
Reference
Gruzdev, I.V.Alferova, M.V.Kondratenok, B.M.Zenkevich, I.G.Quantification of chloroanilines in drinking water by gas chromatography as bromo derivativesRus. J. Anal. Chem.2011, 66, 10, 955-962.
1.Synthesis Route
2.Synthesis Route
3.Synthesis Route
Related Compound Information
Copyright © 2015 MOLBASE All Rights Reserved.
ICP Shanghai 14014220