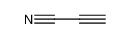-
but-2-ynedinitrile
CAS No.1071-98-3
Formula:C4N2
Dicyanoacetylene, also called carbon subnitride or but-2-ynedinitrile (IUPAC), is a compound of carbon and nitrogen with chemical formula C4N2. It has a linear molecular structure, N≡C−C≡C−C≡N (often abbreviated as NC4N), with alternating triple and single covalent bonds. It can be viewed as acetylene with the two hydrogen atoms replaced by cyanide groups.
At room temperature, dicyanoacetylene is a clear liquid. Because of its high endothermic heat of formation, it can explode to carbon powder and nitrogen gas, and it burns in oxygen with a bright blue-white flame at a temperature of 5260 K (4990 °C, 9010 °F), which is the hottest flame of any known chemical reaction.
2-butynedinitrile
butynedinitrile
di-cyanoacetylene
Dicyanoethyne
Sous-azote de carbone [French]
ACETYLENEDICARBONITRILE
acetylenedicarboxylic acid dinitrile
Sous-azote de carbone
Butindinitril
2-Butynedinitrile (9CI)
expand collapse
Related Compound Information
Copyright © 2015 MOLBASE All Rights Reserved.
ICP Shanghai 14014220