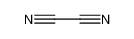1.Identification
1.1 GHS Product identifier
| Product name | hydrogen cyanide |
|---|
1.2 Other means of identification
| Product number | - |
|---|---|
| Other names | Hydrocyanic acid |
1.3 Recommended use of the chemical and restrictions on use
| Identified uses | For industry use only. The major uses of hydrogen cyanide are as an intermediate in the production of a number of chemicals and as an insecticide for fumigating enclosed spaces. Hydrogen cyanide has also been used in gas chamber executions. The two most important uses of other cyanide compounds are in electroplating and metal treatment. |
|---|---|
| Uses advised against | no data available |
1.4 Supplier's details
| Company | MOLBASE (Shanghai) Biotechnology Co., Ltd. |
|---|---|
| Address | Floor 4 & 5, Building 12, No. 1001 North Qinzhou Road, Xuhui District, Shanghai, China |
| Telephone | +86(21)64956998 |
| Fax | +86(21)54365166 |
1.5 Emergency phone number
| Emergency phone number | +86-400-6021-666 |
|---|---|
| Service hours | Monday to Friday, 9am-5pm (Standard time zone: UTC/GMT +8 hours). |
2.Hazard identification
2.1 Classification of the substance or mixture
no data available
2.2 GHS label elements, including precautionary statements
| Pictogram(s) | no data available |
|---|---|
| Signal word | no data available |
| Hazard statement(s) | no data available |
| Precautionary statement(s) | |
| Prevention | no data available |
| Response | no data available |
| Storage | no data available |
| Disposal | no data available |
2.3 Other hazards which do not result in classification
no data available
3.Composition/information on ingredients
3.1 Substances
| Chemical name | Common names and synonyms | CAS number | EC number | Concentration |
|---|---|---|---|---|
| hydrogen cyanide | hydrogen cyanide | 74-90-8 | none | 100% |
4.First-aid measures
4.1 Description of necessary first-aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
Fresh air, rest. Half-upright position. No mouth-to-mouth artificial respiration. Administration of oxygen may be needed. Refer for medical attention. See Notes.
In case of skin contact
Rinse skin with plenty of water or shower. Refer for medical attention . Wear protective gloves when administering first aid.
In case of eye contact
First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention.
If swallowed
Rinse mouth. Do NOT induce vomiting. NO mouth-to-mouth artificial respiration. Administration of oxygen may be needed. Refer for medical attention . See Notes.
4.2 Most important symptoms/effects, acute and delayed
Excerpt from ERG Guide 154 [Substances - Toxic and/or Corrosive (Non-Combustible)]: TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. (ERG, 2016)
Excerpt from ERG Guide 117 [Gases - Toxic - Flammable (Extreme Hazard)]: TOXIC; Extremely Hazardous. May be fatal if inhaled or absorbed through skin. Initial odor may be irritating or foul and may deaden your sense of smell. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution. (ERG, 2016)
It is super toxic. Breathing in a small amount of the gas or swallowing a very small amount may be fatal. Average fatal dose is 50-60 mg. A few minutes of exposure to 300 ppm may result in death. Exposure to 150 ppm for 1/2 to 1 hour may endanger life. (EPA, 1998)
Excerpt from ERG Guide 117 [Gases - Toxic - Flammable (Extreme Hazard)]: TOXIC; Extremely Hazardous. May be fatal if inhaled or absorbed through skin. Initial odor may be irritating or foul and may deaden your sense of smell. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution. (ERG, 2016)
Excerpt from ERG Guide 152 [Substances - Toxic (Combustible)]: Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. (ERG, 2016)
Excerpt from ERG Guide 131 [Flammable Liquids - Toxic]: TOXIC; may be fatal if inhaled, ingested or absorbed through skin. Inhalation or contact with some of these materials will irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. (ERG, 2016)
4.3 Indication of immediate medical attention and special treatment needed, if necessary
/PREHOSPITAL/ Management of cyanide poisoning begins with removal to fresh air. Dermal decontamination is unnecessary if exposure has been only to vapor, but wet clothing should be removed and the underlying skin should be washed with soap and water or water alone if liquid on the skin is a possibility. Attention to the basics of intensive supportive care is critical and includes mechanical ventilation as needed, circulatory support with crystalloids and vasopressors, correction of metabolic acidosis with IV sodium bicarbonate, and seizure control with benzodiazepine administration. ... Administration of 100% oxygen has been found empirically to exert a beneficial effect and should be a part of general supportive care for every cyanide-poisoned patient. /Cyanides/
5.Fire-fighting measures
5.1 Extinguishing media
Suitable extinguishing media
Fire situation may require evacuation. Allow burning of material until flow of gas can be stopped. Use water spray, dry chemical, "alcohol resistant" foam, or carbon dioxide. Water may be ineffective. Approach fire from upwind. Fight fire from protected location or maximum possible distance.
5.2 Specific hazards arising from the chemical
Excerpt from ERG Guide 154 [Substances - Toxic and/or Corrosive (Non-Combustible)]: Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. For electric vehicles or equipment, ERG Guide 147 (lithium ion batteries) or ERG Guide 138 (sodium batteries) should also be consulted. (ERG, 2016)
Excerpt from ERG Guide 117 [Gases - Toxic - Flammable (Extreme Hazard)]: These materials are extremely flammable. May form explosive mixtures with air. May be ignited by heat, sparks or flames. Vapors from liquefied gas are initially heavier than air and spread along ground. Vapors may travel to source of ignition and flash back. Runoff may create fire or explosion hazard. Cylinders exposed to fire may vent and release toxic and flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket. (ERG, 2016)
Unstabilized hydrocyanic acid may polymerize spontaneously with explosive violence. Flashback along vapor trail may occur. The explosion hazard is severe when this material is exposed to heat, flame, or oxidizers. It forms explosive mixtures with air, and will react with water, steam, acid, or acid fumes to produce highly toxic fumes of cyanides. It may decompose explosively upon contact with alkaline material. Avoid acetylaldehyde, alkaline materials, oxidizers, water, steam, acid, and acid fumes. Hydrocyanic acid solution is sensitive to light. It may become unstable and subject to explosion if stored for an extended time or exposed to high temperature and pressure. Avoid heat, flame or oxidizers. Hazardous polymerization may occur. Unstabilized hydrocyanic acid may polymerize spontaneously with explosive violence. Can polymerize at 122-140F or when catalyzed with traces of alkali. (EPA, 1998)
Excerpt from ERG Guide 117 [Gases - Toxic - Flammable (Extreme Hazard)]: These materials are extremely flammable. May form explosive mixtures with air. May be ignited by heat, sparks or flames. Vapors from liquefied gas are initially heavier than air and spread along ground. Vapors may travel to source of ignition and flash back. Runoff may create fire or explosion hazard. Cylinders exposed to fire may vent and release toxic and flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket. (ERG, 2016)
Excerpt from ERG Guide 152 [Substances - Toxic (Combustible)]: Combustible material: may burn but does not ignite readily. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form. (ERG, 2016)
Excerpt from ERG Guide 131 [Flammable Liquids - Toxic]: HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion and poison hazard indoors, outdoors or in sewers. Those substances designated with a (P) may polymerize explosively when heated or involved in a fire. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. (ERG, 2016)
5.3 Special protective actions for fire-fighters
Wear self-contained breathing apparatus for firefighting if necessary.
6.Accidental release measures
6.1 Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust. For personal protection see section 8.
6.2 Environmental precautions
Evacuate danger area! Consult an expert! Personal protection: gas-tight chemical protection suit including self-contained breathing apparatus. Do NOT let this chemical enter the environment. Ventilation. Remove all ignition sources. Absorb remaining liquid in sand or inert absorbent. Then store and dispose of according to local regulations. NEVER direct water jet on liquid.
6.3 Methods and materials for containment and cleaning up
1. Remove all ignition sources. 2. Ventilate area of spill or leak. 3. If in gaseous form, stop flow of gas. If source of leak is cylinder & leak cannot be stopped in place, remove ... to safe place in open air ... repair leak or allow cylinder to empty.
7.Handling and storage
7.1 Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols. Avoid exposure - obtain special instructions before use.Provide appropriate exhaust ventilation at places where dust is formed. For precautions see section 2.2.
7.2 Conditions for safe storage, including any incompatibilities
Fireproof. Separated from food and feedstuffs. Cool. Store only if stabilized.Keep cylinders of hydrogen cyanide (HCN) cool and away from open flames. Make certain that HCN cylinders are adequately supported and grounded during storage and emptying. Store cylinders in a vertical position. Do not drop cylinders or damage them by impact. Cylinders must be returned to the supplier within 90 days of the filling date marked on the cylinders, regardless of whether or not the contents have been used. This is due to the possibility of HCN becoming unstable over time. If there is any indication that the HCN is becoming unstable, such as a darkening of the product or an increase in cylinder pressure, contact the supplier immediately for instructions.
8.Exposure controls/personal protection
8.1 Control parameters
Occupational Exposure limit values
Recommended Exposure Limit: 15 Min Short-Term Exposure Limit: 4.7 ppm (5 mg/cu m). Skin.
Biological limit values
no data available
8.2 Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday.
8.3 Individual protection measures, such as personal protective equipment (PPE)
Eye/face protection
Safety glasses with side-shields conforming to EN166. Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Wear impervious clothing. The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique(without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it.
Respiratory protection
Wear dust mask when handling large quantities.
Thermal hazards
no data available
9.Physical and chemical properties
| Physical state | colourless or pale blue liquid, or colourlessgas, (depending upon temperature) with a bitter almond odour |
|---|---|
| Colour | Colorless gas or liquid |
| Odour | Characteristic sweetish, like almond |
| Melting point/ freezing point | -14ºC |
| Boiling point or initial boiling point and boiling range | 25 - 26ºC |
| Flammability | Extremely flammable. Gives off irritating or toxic fumes (or gases) in a fire. |
| Lower and upper explosion limit / flammability limit | Lower flammable limit: 5.6% by volume; Upper flammable limit: 40.0% by volume |
| Flash point | 17.5ºC |
| Auto-ignition temperature | 540°C (USCG, 1999) |
| Decomposition temperature | no data available |
| pH | Very weak acid (does not redden litmus) |
| Kinematic viscosity | no data available |
| Solubility | In water:insoluble |
| Partition coefficient n-octanol/water (log value) | log Kow = -0.25 |
| Vapour pressure | 630 mm Hg (EPA, 1998) |
| Density and/or relative density | 0.69 |
| Relative vapour density | 0.901 (EPA, 1998) (Relative to Air) |
| Particle characteristics | no data available |
10.Stability and reactivity
10.1 Reactivity
no data available
10.2 Chemical stability
SOLN SENSITIVE TO LIGHT
10.3 Possibility of hazardous reactions
Flammable and dangerous fire hazard ... May be ignited by fires, heated materials, and sparks.The gas mixes well with air, explosive mixtures are easily formed.Hazardous concentrations may develop quickly in enclosed or poorly-ventilated areas.Hydrogen cyanide (AC) gas mixes well with air; explosive mixtures are easily formed.HYDROCYANIC ACID, AQUEOUS SOLUTION, WITH NOT MORE THAN 20% HYDROGEN CYANIDE reacts with acid to evolve hydrogen cyanide, a very poisonous colorless gas smelling of bitter almonds which is a deadly human poison by all routes. Carbon dioxide from the air is sufficiently acidic to liberate HCN from aqueous solutions of hydrocyanic acid [Lewis]. The solution also can evolve gaseous hydrogen cyanide when heated. Inhalation of gaseous HCN is quickly fatal by respiratory arrest. The gas forms flammable or explosive mixtures with air (may be difficult to ignite at lower concentrations). It presents an explosion hazard when heated with or exposed to other oxidizing agents and may polymerize explosively at elevated temperature (50-60°C) or in the presence of traces of alkali [Wohler, L. et al., Chem. Ztg., 1926, 50, p. 761, 781]. It reacts violently with acetaldehyde. During the preparation of imidoester hydrochlorides, hydrogen chloride was rapidly passed over an alcoholic solution of hydrogen cyanide. An explosion ensued, despite cooling of the process [J. Org. Chem., 1955, 20, 1573]. In the absence of a stabilizer (e.g. phosphoric acid) it may undergo explosively rapid spontaneous (autocatalytic) polymerization leading to a fire. The reaction is autocatalytic because of ammonia formation [Bond, J., Loss Prev. Bull., 1991, 101, p.3].
10.4 Conditions to avoid
no data available
10.5 Incompatible materials
Unless stabilized and maintained, samples stored more than 90 days are hazardous. The gas can form explosive mixtures with air. Material containing more than 2-5% water are less stable than dry material and can be self-reactive, forming explosive mixtures with air. Heat above 50-60°C or contact with amines or strong bases can cause polymerization. ... attacks some plastics, rubber, and coatings.
10.6 Hazardous decomposition products
Extremely toxic vapors /unspecified/ are generated even at ordinary temperatures.
11.Toxicological information
Acute toxicity
- Oral: LD50 Mouse oral 3700 ug/kg
- Inhalation: LC50 Rat inhalation 3778 mg/cu m (10 sec)
- Dermal: no data available
Skin corrosion/irritation
no data available
Serious eye damage/irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
EPA-II
Reproductive toxicity
No studies were located on the reproductive or developmental effects of cyanide in humans from inhalation exposure. Animal studies have suggested that oral exposure to cassava (a cyanide-containing vegetable) may be associated with malformations in the fetus and low fetal body weights.
STOT-single exposure
no data available
STOT-repeated exposure
no data available
Aspiration hazard
no data available
12.Ecological information
12.1 Toxicity
- Toxicity to fish: LC50 Lepomis macrochirus (bluegill swim up fry) 232-365 ug/L/96 hr, flow-through bioassay
- Toxicity to daphnia and other aquatic invertebrates: no data available
- Toxicity to algae: no data available
- Toxicity to microorganisms: no data available
12.2 Persistence and degradability
Waste water treatment; sludge digestion: at 25 mg/L: no adverse effect in 24 days; at 30 mg/L: initial retarding effect for 6 days; at 50 mg/L: 10% reduction in gas production.
12.3 Bioaccumulative potential
An estimated BCF of 3 was calculated in fish for hydrogen cyanide(SRC), using log Kow of -0.25(1) and a regression-derived equation(2). According to a classification scheme(3), this BCF suggests the potential for bioconcentration in aquatic organisms is low(SRC).
12.4 Mobility in soil
Hydrogen cyanide is only weakly absorbed by organic matter(1). Hydrogen cyanide is not strongly partitioned into the sediments or suspended adsorbents, primarily due to its high solubility in water. Cyanide mobility is least where soils exhibit low pH, high concn of free iron oxides, and positively charged particles (eg, kaolin, chlorite, gibbsite). Mobility is greatest at high pH, high concn of free calcium carbonate (high negative charge) and low clay content. Adsorption of hydrogen cyanide by montmorillonitic clays is fairly weak and is decreased by the presence of water(2).
12.5 Other adverse effects
no data available
13.Disposal considerations
13.1 Disposal methods
Product
The material can be disposed of by removal to a licensed chemical destruction plant or by controlled incineration with flue gas scrubbing. Do not contaminate water, foodstuffs, feed or seed by storage or disposal. Do not discharge to sewer systems.
Contaminated packaging
Containers can be triply rinsed (or equivalent) and offered for recycling or reconditioning. Alternatively, the packaging can be punctured to make it unusable for other purposes and then be disposed of in a sanitary landfill. Controlled incineration with flue gas scrubbing is possible for combustible packaging materials.
14.Transport information
14.1 UN Number
| ADR/RID: UN3265 | IMDG: UN3265 | IATA: UN3265 |
14.2 UN Proper Shipping Name
| ADR/RID: CORROSIVE LIQUID, ACIDIC, ORGANIC, N.O.S. |
| IMDG: CORROSIVE LIQUID, ACIDIC, ORGANIC, N.O.S. |
| IATA: CORROSIVE LIQUID, ACIDIC, ORGANIC, N.O.S. |
14.3 Transport hazard class(es)
| ADR/RID: 8 | IMDG: 8 | IATA: 8 |
14.4 Packing group, if applicable
| ADR/RID: II | IMDG: II | IATA: II |
14.5 Environmental hazards
| ADR/RID: no | IMDG: no | IATA: no |
14.6 Special precautions for user
no data available
14.7 Transport in bulk according to Annex II of MARPOL 73/78 and the IBC Code
no data available
15.Regulatory information
15.1 Safety, health and environmental regulations specific for the product in question
| Chemical name | Common names and synonyms | CAS number | EC number |
|---|---|---|---|
| hydrogen cyanide | hydrogen cyanide | 74-90-8 | none |
| European Inventory of Existing Commercial Chemical Substances (EINECS) | Listed. | ||
| EC Inventory | Listed. | ||
| United States Toxic Substances Control Act (TSCA) Inventory | Listed. | ||
| China Catalog of Hazardous chemicals 2015 | Listed. | ||
| New Zealand Inventory of Chemicals (NZIoC) | Listed. | ||
| Philippines Inventory of Chemicals and Chemical Substances (PICCS) | Listed. | ||
| Vietnam National Chemical Inventory | Not Listed. | ||
| Chinese Chemical Inventory of Existing Chemical Substances (China IECSC) | Listed. | ||
16.Other information
Information on revision
| Creation Date | Aug 14, 2017 |
|---|---|
| Revision Date | Aug 14, 2017 |
Abbreviations and acronyms
- CAS: Chemical Abstracts Service
- ADR: European Agreement concerning the International Carriage of Dangerous Goods by Road
- RID: Regulation concerning the International Carriage of Dangerous Goods by Rail
- IMDG: International Maritime Dangerous Goods
- IATA: International Air Transportation Association
- TWA: Time Weighted Average
- STEL: Short term exposure limit
- LC50: Lethal Concentration 50%
- LD50: Lethal Dose 50%
- EC50: Effective Concentration 50%
References
- IPCS - The International Chemical Safety Cards (ICSC), website: http://www.ilo.org/dyn/icsc/showcard.home
- HSDB - Hazardous Substances Data Bank, website: https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm
- IARC - International Agency for Research on Cancer, website: http://www.iarc.fr/
- eChemPortal - The Global Portal to Information on Chemical Substances by OECD, website: http://www.echemportal.org/echemportal/index?pageID=0&request_locale=en
- CAMEO Chemicals, website: http://cameochemicals.noaa.gov/search/simple
- ChemIDplus, website: http://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
- ERG - Emergency Response Guidebook by U.S. Department of Transportation, website: http://www.phmsa.dot.gov/hazmat/library/erg
- Germany GESTIS-database on hazard substance, website: http://www.dguv.de/ifa/gestis/gestis-stoffdatenbank/index-2.jsp
- ECHA - European Chemicals Agency, website: https://echa.europa.eu/